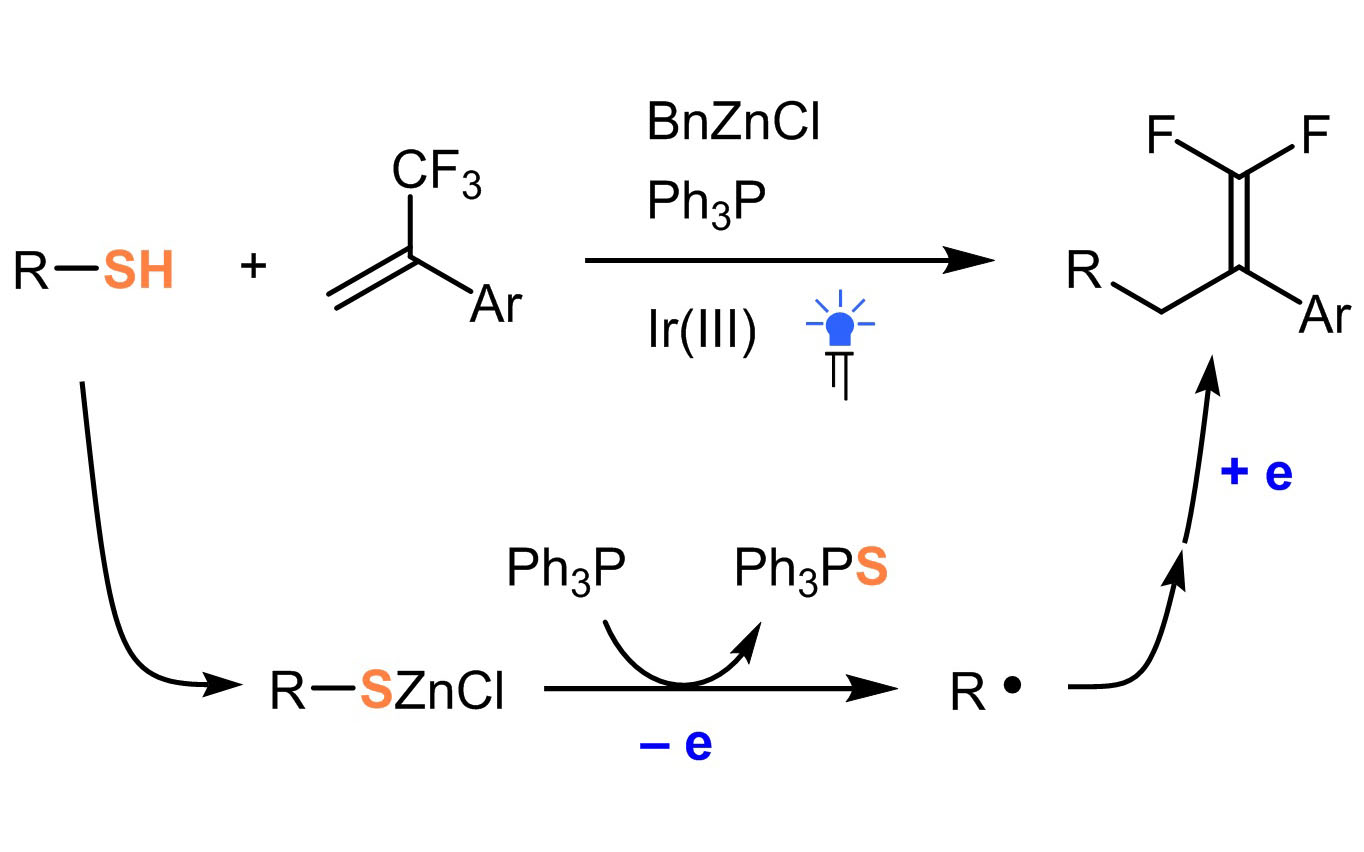
Ученые ИОХ РАН разработали метод генерации алкильных радикалов из тиолов
Свободнорадикальные превращения находят широкое применение в органической химии. Генерация углерод-центрированных радикалов чаще всего происходит из соответствующих галогенированных производных. В последнее время значительное внимание исследователей привлекают процессы генерации C-центрированных радикалов путем гомолитического разрыва связи C-S. Генерация таких интермедиатов непосредственно из тиолов является довольно проблематичной по причине протекания побочного процесса переноса атома водорода.
Исследователям ИОХ РАН под руководством д.х.н. А.Д. Дильмана удалось осуществить эффективный процесс генерации C-центрированных алкильных радикалов из тиолов, а также вовлечь их в реакцию с алкенами. Успех обеспечивается insituгенерированием тиолятов цинка, которые являются непосредственными предшественниками радикалов. Это позволяет исключить нахождение свободного тиола в реакционной смеси и, как следствие, побочный процесс переноса атома водорода на возникающий радикал не происходит.
Источник:
Vyacheslav I. Supranovich, Vitalij V. Levin, Vladimir A. Kokorekin, Alexander D. Dilman Generation of Alkyl Radicals from Thiols via Zinc Thiolates: Application for the Synthesis of gem-Difluorostyrenes Adv. Synth. Catal., 2021, accepted manuscript. DOI: 10.1002/adsc.202100088.