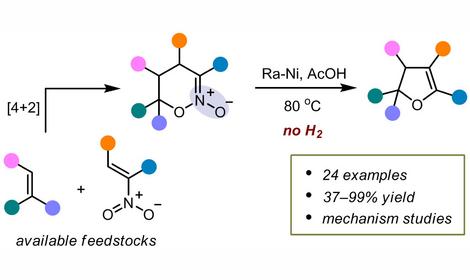
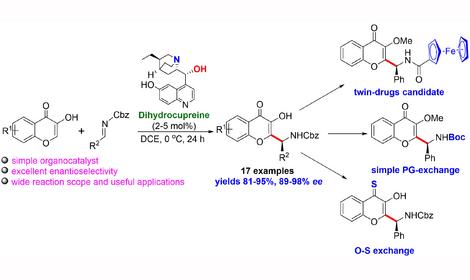
Laboratory of Directed Functionalization of Organic Molecular Systems (№ 33)

Head of the Laboratory: Prof., Dr. I.V. Trushkov, e-mail: itrushkov@mail.ru
The main research areas of the Laboratory:
- Chemistry of donor-acceptor alkenes and cyclopropanes;
- Development of methods for the synthesis of rare classes of functionalized aza- and polyazaheterocyclic compounds (with different numbers of heteroatoms, different ring sizes, different degrees of saturation, monocyclic and condensed, etc.) based on the chemistry of carbonic acid amides, as well as their thioxo- and imino analogs;
- Study of the structure and reactivity of synthesized azaheterocycles; finding directions for their practical use;
- Protonic ionic liquids in organic synthesis.
Publications:
Prof. Dr. A.D. Shutalev group:
1. A. A. Fesenko, M. S. Grigoriev, A. D. Shutalev, A convenient stereoselective access to novel 1,2,4-triazepan-3-ones/thiones via reduction or reductive alkylation of 7-membered cyclic semicarbazones and thiosemicarbazones. Org. Biomol. Chem.2018, 16, № 43, 8072-8089. doi: 10.1039/c8ob01766b.
2. A. A. Fesenko, A. D. Shutalev, A General Stereoselective Approach to 1,2,4-Triazepan-3-thiones/ones via Reduction or Reductive Alkylation of 2,4,5,6-Tetrahedro-3H-1,2,4-triazepine-3-thiones/ones. Proceedings2019, 9, № 1, 14. doi: 10.3390/ecsoc-22-05683.
3. P. A. Solovyev, A. A. Fesenko, A. D. Shutalev, A New Approach to 5-Functionalized 1,2-Dihydropyrimidin-2-ones/imines via Base-Induced Chloroform Elimination from 4-Trichloromethyl-1,2,3,4-tetrahydropyrimidin-2-ones/imines. Proceedings2019, 9, № 1, 15. doi: 10.3390/ecsoc-22-05678.
4. A. A. Fesenko, L. A. Trafimova, M. O. Zimin, A. S. Kuvakin, A. D. Shutalev, A New Efficient Route to 2-AlkylSemicarbazides. Proceedings2019, 41, № 1, 46. doi: 10.3390/ecsoc-23-06501.
5. A. A. Fesenko, L. A. Trafimova, M. O. Zimin, A. S. Kuvakin, A. D. Shutalev, N2-Alkylation of semicarbazones. A general and efficient protocol for the synthesis of 2-alkylsemicarbazides from semicarbazide. Arkivoc, 2019, ii, 176-189. doi: 10.24820/ark.5550190.p011.024.
6. A. A. Fesenko, A, N. Yankov, A. D. Shutalev, A general and convenient synthesis of 4-(tosylmethyl)semicarbazones and their use in amidoalkylation of hydrogen, heteroatom, and carbon nucleophiles. Tetrahedron2019, 75, № 45, 130527. doi: 10.1016/j.tet.2019.130527.
7. A. A. Fesenko, A. D. Shutalev, Different pathways in the reaction of N-(tosylmethyl)-substituted ureas, thioureas, and N'-cyanoguanidines with sodium cyanide. Synthesis of α-ureido nitriles, α-ureido amides, and hydantoin imino derivatives. Tetrahedron2020, 76, 131340. doi: 10.1016/j.tet.2020.131340.
8. A. Dobrov, A. Fesenko, A. Yankov, I. Stepanenko, D. Darvasiova, M. Breza, P. Rapta, L. M. D. R. S. Martins, A. J. L. Pombeiro, A. Shutalev, V. B. Arion, Inorg. Chem. in press, doi: 10.1021/acs.inorgchem.0c01119.
Prof. Dr. I.V. Trushkov group:
1. I. V. Trushkov, M. G. Uchuskin, V. T. Abaev, O. V. Serdyuk. Indolylvinyl Ketones: Building Blocks for the Synthesis of Natural Products and Bioactive Compounds. Synthesis 2019, 51, 787-815.doi: 10.1055/s-0037-1611702.
2. А. А. Merkushev, I. V. Trushkov, М. G. Uchuskin. Modern methods for the synthesis ofd-carbolines. Russ. Chem. Bull. 2019,68, № 4, 681-690. doi: 10.1007/s11172-019-2475-6.
3. A. O. Chagarovskiy, E. D. Strel’tsova, V. B. Rybakov, I. I. Levina, I. V. Trushkov. Synthesis of 2.3-diaryl-2,3,4,4a-tetrahydro-5H-indeno[1,2-c]pyridazin-5-ones. Chem. Heterocycl. Comp.2019, 55, № 3, 240-245.doi: 10.1007/s10593-019-02448-y.
4. Boichenko M. A., Babkin I. Yu., Kobylskoy S. G., Chagarovskiy A. O., Ivanova O. A., Trushkov I. V. 4b,5,6,9-Tetrahydro-7H-dibenzo[c,e]pyrrolo[1,2-a]azepin-7-one. Molbank 2019, 2019(2), M1061. doi: 10.3390/M1061.
5. A. O. Chagarovskiy, V. V. Kuznetsov, O. A. Ivanova, A. S. Goloveshkin, I. I. Levina, N. N. Makhova, I. V. Trushkov Synthesis of 1-Substituted Pyrazolines by Reaction of Donor-Acceptor Cyclopropanes with 1,5-Diazabicyclo[3.1.0]hexanes Eur. J. Org. Chem. – 2019. – № 31-32. – p. 5475-5485. doi: 10.1002/ejoc.201900579.
6. Makarov A. S., Kekhvaeva A. E., Chalikidi P. N., Abaev V. T., Trushkov I. V., Uchuskin M. G. A Simple Synthesis of Densely Substituted Benzofurans by Domino Reaction of 2-Hydroxybenzyl Alcohols with 2-Substituted Furans. Synthesis2019, 51, 3747-3757. doi: 10.1055/s-0039-1690000.
7. Zelina E. Y., Nevolina T. A., Skvortsov D. A., Trushkov I. V., Uchuskin M. G. A Route to (Het)arene-Annulated Pyrrolo[1,2-d][1,4]diazepines via the Expanded Paal-Knorr Reaction: Nitro Group and Furan Ring as Equivalents of Amino Group and 1,4-Diketone. J. Org. Chem.2019, 84, 13707-13720. doi: 10.1021/acs.joc.9b01925.
8. Ivanova O. A., Trushkov I. V. Donor-Acceptor Cyclopropanes in the Synthesis of Carbocycles. Chem. Rec.2019, 19, № 11, 2189-2208.doi: 10.1002/tcr.201800166.
9. BoichenkoM. A., ChagarovskiyA. O., RybakovV. B., TrushkovI. V., IvanovaO. A. Dimethyl 2-{ [2-(2-Methoxy-1-methoxycarbonyl-2-oxoethyl)-4,5,7-trimethoxy-3-(2,4,5-trimethoxyphenyl)-2,3-dihydro-1H-inden-1-yl]methyl}malonate. Molbank 2020, M1107, doi: 10.3390/M1107.
10. Boichenko M. A., Andreev I. A., Chagarovskiy A. O., Levina I. I., Zhokhov S. S., Trushkov I. V., Ivanova O. A. Ring Opening of Donor–Acceptor Cyclopropanes with Cyanide Ion and Its Surrogates. J. Org. Chem.2020, 85, №2, 1146-1157. doi: 10.1021/acs.joc.9b03098.
11. Nguyen H. M., Chand H. R., Golantsov N. E., Trushkov I. V., Voskressensky L. G. Cyclopentene Assembly by Microwave-Assisted Domino Reaction of Donor-Acceptor Cyclopropanes with Ketals. Synlett 2020, 31, n3, 295-299. doi: 10.1055/s-0039-1690775.
12. Zelina E. Yu., Nevolina T. A., Sorotskaja L. N., Skvortsov D. A., Trushkov I. V., Uchuskin M. G. Route to pyrrolo[1,2-a]quinaxolinesvia a furan ring opening–pyrrole ring closure sequence. Tetrahedron Lett. 2020, 61, 151532. doi:10.1016/j.tetlet.2019.151532.
13. Ratmanova N. K., Andreev I. A., Trushkov I. V. Methods for the synthesis of plerixafor. Chem. Heterocycl. Comp.2020, 56, №1, 30-35.doi: 10.1007/s10593-020-02617-4.
14. Ratmanova N. K., Andreev I. A., Leontiev A. V.; Momotova D., Novoselov A. M.; Ivanova O. A., Trushkov I. V. Strategic approaches to the synthesis of pyrrolizidine and indolizidine alkaloids. Tetrahedron 2020, 76, n14, 131031. doi: 10.1016/j.tet.2020.131031.
15. Kuznetsova L. I., Chagarovskiy A. O., Levina I. I., Rybakov V. B., Ivanova O. A., Trushkov I. V. A simple synthesis of 2-{ 2-[(arylmethylidene)amino]indazol-3-yl}malonate esters. Chem. Heterocycl. Compd. 2020, 56, № 5, 555-561.doi: 10.1007/s10593-020-02699-0.