Laboratory of Medicinal Chemistry (N17)

Head: Victor V. Semenov, DSc (Chem.) (e-mail: vsioc.ac.ru, tel.: +7(499) 135-6343)
The laboratory was established in 1961 and headed by Dr. N.F. Kononov (1961-1979) and by Prof. S.Z. Taits (1980-1989).
Main achievements of the laboratory
1. For the needs of medicinal chemistry, a technology for the isolation of valuable intermediates of allylpolyalkoxybenzenes from available natural raw materials — parsley and dill seeds using extraction with liquid CO2 and highly efficient rectification has been developed. These crops, prepared on a large scale using well-established cultivation and harvesting technology, are more attractive and cheaper than wild-growing ones. The extracts are produced at the factory of the Caravan company (Belozerny village, Krasnodar).
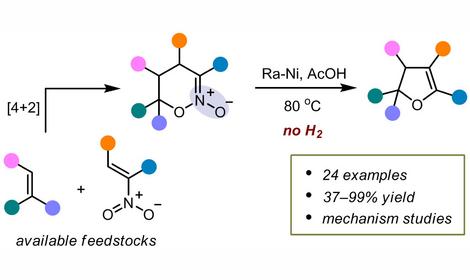
2. More than 200 analogues of natural antimitotics — cell division inhibitors: combretastatin, podophyllotoxin, steganocin, glazovianin A and others have been synthesized from natural allylbenzenes, isolated from industrial extracts of parsley and dill seeds. At the moment these compounds are at different stages of clinical trials for chemotherapy of cancer in the USA , Japan and Europe.
3. Almost all natural tubulin polymerization inhibitors are found in plants distributed outside of Russia, mainly in Africa and America, and previously were not widely available. Their mechanism of action is considered with a disturbance of the tubulin polymerization. This protein is responsible for the formation of the mitotic spindle, which ensures the distribution of chromosomes between two daughter cells in the process of cell division. Together with the N. K. Koltzov Institute of Developmental Biology of Russian Academy of Sciences, an original method for the detection of tubulin polymerization inhibitors on a model of sea urchin embryos, which allows testing of up to 40-50 molecules per month, was developed.
4. In cooperation with the A. I. Burnasyan Federal Medical Biophysical Center of Federal Medical Biological Agency the laboratory-scale production of ligands to obtain 99mTc-containing radiopharmaceuticals, used in clinics from available Russian raw materials with high conversion of the starting reagents, was organized. This made it possible to completely abandon the import supplies of these ligands and significantly reduce their cost.
5. A constantly updated collection of chemical compounds (about 200,000) has been created for screening for various types of biological activity. A program has been developed for recording these compounds in the warehouse and on-line searching for both molecules and their NMR and mass spectra by structural fragment.
6. As part of a joint humanitarian project with the University of Queensland (Brisbane, Australia) on the search for antimicrobials, 150,000 new molecules were investigated and more than 3,000 active molecules of interest for further research were found.
7. The laboratory uses original Russian ozonation and hydrogenation devices. The ozonizer is equipped with an infrared ozone concentration sensor with an electronic system, which generates a fixed amount of ozone. It was shown that highly porous block cellular hydrogenation catalysts could be repeatedly (up to 30–40 times) regenerated directly in the reactor at 400 °C after each hydrogenation without significant loss of activity. Due to high porosity and strength of the catalyst, the reaction mixture can be quickly removed from the reactor without the use of filter.
In scientific and technical developments, the laboratory uses the services of leading world companies and universities: GlaxoSmithKline (Great Britain), Novartis (Basel, Switzerland), Immune Pharmaceuticals LLC (New York, USA), National Cancer Institute (Bethesda, USA), University of Queensland (Brisbane, Australia) University of Auckland (Auckland, New Zealand), University of Pisa (Pisa, Italy), etc.
Selected publications:
1. E. A. Silyanova, A. V. Samet, L. K. Salamandra, V. N. Khrustalev, V. V. Semenov. Formation of 3,4-Diarylpyrrole- and Pyrrolocoumarin Core of Natural Marine Products via Barton-Zard Reaction and Selective O-demethylation. Eur. J. Org. Chem., 2020, in press. DOI: 10.1002/ejoc.202000099
2. A.V.Samet, O. G. Shevchenko, V. V. Rusak, E. M. Chartov, A. B. Myshlyavtsev, D. A. Rusanov, M. N. Semenova, V. V. Semenov. Antioxidant Activity of Natural Allylpolyalkoxybenzene Plant Essential Oil Constituents. J. Nat. Prod., 2019, 82, 1451–1458. DOI:10.1021/acs.jnatprod.8b00878
3. A. S. Maksimenko, V. P. Kislyi, N. B. Chernysheva, Yu. A. Strelenko, Y. V. Zubavichus, V. N. Khrustalev, M. N. Semenova, V.V. Semenov. Effective synthesis of 3,4-diaryl-isoxazole-5-carboxamides and their antiproliferative properties. Eur. J. Org. Chem., 2019,2019,4260- 4270. DOI: 10.1002/ejoc.201900643
4. B. V. Lichitsky, A. N. Komogortsev, A. N. Dudinov, M. M. Krayushkin, E. N. Khodot, A. V. Samet, E. A. Silyanova, L. D. Konyushkin, A. S. Karpov, D. Gorses, T. Radimerski,M. N. Semenova, A. S. Kiselyov, V. V.Semenov. Benzimidazolyl-pyrazolo[3,4-b]pyridinones — selective inhibitors of MOLT-4 leukemia cell growth and sea urchin embryo spiculogenesis: Target quest.
ACS Comb. Sci., 2019, 21, 805-816. DOI: 10.1021/acscombsci.9b00135.
5. M. N. Semenova, D. V. Demchuk, D. V. Tsyganov, N. B. Chernysheva, A. V. Samet, E. A. Silyanova, V. P. Kislyi, A. S. Maksimenko, A. E. Varakutin, L. D. Konyushkin, M. M. Raihstat, A. S. Kiselyov, V. V. Semenov. The sea urchin embryo model as a reliable in vivo phenotypic screen to characterize selective antimitotic molecules. Comparative evaluation of combretapyrazoles, -isoxazoles, -1,2,3-triazoles, and -pyrroles as tubulin binding agents. ACS Comb. Sci., 2018, 20, 700-721. DOI: 10.1021/acscombsci.8b00113
6. N. B. Chernysheva, A. S. Maksimenko, F. A. Andreyanov, V. P. Kislyi, Yu. A. Strelenko, V. N. Khrustalev, M. N. Semenova, V. V. Semenov. Regioselective synthesis of 3,4-diaryl-5-unsubstituted isoxazoles, analogues of natural cytostatic combretastatin A4. Eur. J. Med. Chem., 2018, 146, 511-518. DOI: 10.1016/j.ejmech.2018.01.070
7. N. B. Chernysheva, A. S. Maksimenko, F. A. Andreyanov, V. P. Kislyi, Yu. A. Strelenko, V. N. Khrustalev, M. N. Semenova, V. V. Semenov. Synthesis of 3,4-diaryl-5-carboxy-4,5-dihydroisoxazole 2-oxides as valuable synthons for anticancer molecules. Tetrahedron, 2017, 73, 6728-6735. DOI: 10.1016/j.tet.2017.10.016
8. V. V. Semenov, B. V. Lichitsky, A. N. Komogortsev, A. A. Dudinov, M. M. Krayushkin, L. D. Konyushkin, O. P. Atamanenko, I. B. Karmanova, Yu. A. Strelenko, B. Shor, M. N. Semenova, A. S. Kiselyov. Synthesis and anti-mitotic activity of 6,7-dihydro-4H-isothiazolo[4,5-b]pyridin-5-ones: In vivo and cell-based studies. Eur. J. Med. Chem., 2017, 125, 573-585. DOI: 10.1016/j.ejmech.2016.09.075
9. Ch. Eurtivong, V. V. Semenov, M. N. Semenova, L. D. Konyushkin, O. P. Atamanenko, J. Reynisson, A. S. Kiselyov. 3-Amino-thieno[2,3-b]pyridines as microtubule-destabilising agents: Molecular modelling and biological evaluation in the sea urchin embryo and human cancer cells. Bioorg. Med. Chem., 2017, 25, 658–664. DOI: 10.1016/j.bmc.2016.11.041
10. L. Testai, I. Yu. Strobykina, V. V. Semenov, M. Nю Semenova, E. Da Pozzo, A. Martelli, V. Citi, C. Martini, M. C. Breschi, V. E. Kataev, V. Calderone. Mitochondriotropic and Cardioprotective Effects of Triphenylphosphonium-Conjugated Derivatives of the Diterpenoid Isosteviol. Int. J. Mol. Sci., 2017, 18, 2060.· DOI:10.3390/ijms18102060
11. R. Chuprov-Netochin, D. Tsyganov, V. Semenov, M. Semenova, P. Volynchuk, E. Marusich, V. Nazarenko, S. Leonov. In vitro/in vivo antimitotic activity and structure-activity relationships of New Glaziovianin A Isoflavone Series. FEBS Journal, 2016, 283, P-05.01.1-021. DOI: 10.1111/febs.13808
12. V. V. Semenov, B. V. Lichitsky, A. N. Komogortsev, A. A. Dudinov, M. M. Krayushkin, L. D. Konyushkin, O. P. Atamanenko, I. B. Karmanova, Yu. A. Strelenko, B. Shor, M. N. Semenova, A. S. Kiselyov. Synthesis and anti-mitotic activity of 6,7-dihydro-4H-isothiazolo[4,5-b]pyridin-5-ones: In vivo and cell-based studies. Eur. J. Med. Chem., 2017, 125, 573-585. DOI: 10.1016/j.ejmech.2016.09.075
13. V. V. Semenov, D. V. Tsyganov, M. N. Semenova, R. N. Chuprov-Netochin, M. M. Raihstat, L. D. Konyushkin, P. B. Volynchuk, E. I. Marusich, V. V. Nazarenko, S. V. Leonov, A. S. Kiselyov. Efficient Synthesis of Glaziovianin A Isoflavone Series from Dill and Parsley Extracts and Their in Vitro/in Vivo Antimitotic Activity. J. Nat. Prod., 2016, 79, 1429−1438. DOI: 10.1021/acs.jnatprod.6b00173
14. I. B. Karmanova, S. I. Firgang, L. D. Konyushkin, V. N. Khrustalev, A. V. Ignatov, L. A. Kuznetsov, Yu. A. Pinchuk, I. A. Kozlov, V. V. Semenov. Dill and parsley seed extracts in scale up synthesis of aminopolyalkoxybenzenes — Beneficial synthons for fused nitrogen polyalkoxyheterocycles. Mend. Commun., 2016, 26, 66-68. DOI: 10.1016/j.mencom.2016.01.026
15. D. V. Tsyganov, M. M. Krayushkin, L. D. Konyushkin, Yu. A. Strelenko, M. N. Semenova, V. V. Semenov. Facile Synthesis of Natural Alkoxynaphthalene Analogues from Plant Alkoxybenzenes. J. Nat. Prod., 2016, 79, 923-928. DOI: 10.1021/acs.jnatprod.5b01007